Description
New Quantitative Covid-19 antigen test by FIA Genedia Quantum technology
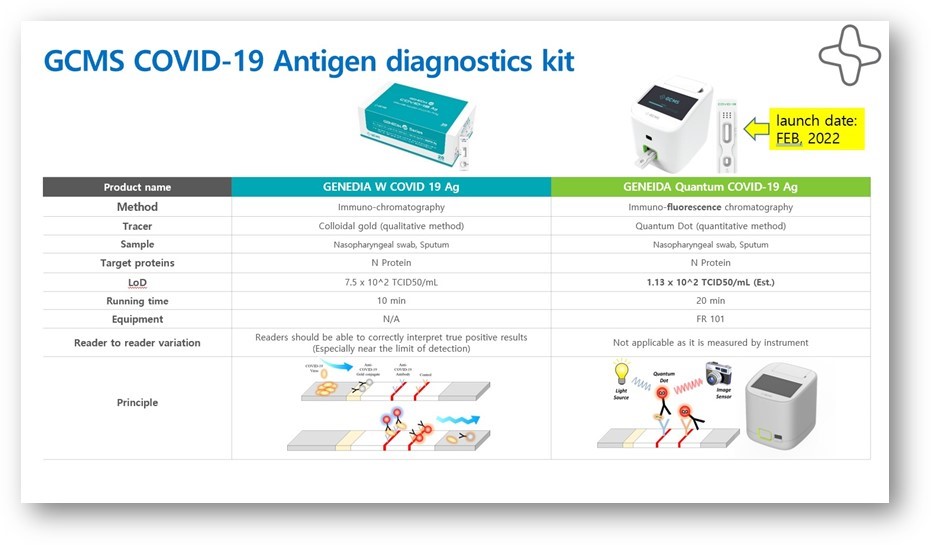
Genedia Quantum is the most advanced system for antigen testing. The kit consists of Quantum COVID-19 Ag tests and the Quantum FR101 analyzer. This innovative FIA (Fluorescence Immunoassay Antigen) technology uses a quantitative immunoassay system based on microfluidic and immunofluorescence technology, similar to the RTPCR type used to measure generation rates.
The Quantum system also uses fluorescence in a revolutionary way since it allows quantifying the amount of the parameters that are being measured, in this case, the antigen or the peak glycoprotein of SARS-CoV2. In less than 15 minutes, the test is able to quantitatively determine the antigen amount or the level of COVID-19 infection of an individual.
With the GENEDIA QUANTUM system, human error is eliminated and superior reliability and accuracy are possible in a short period of time. The result is improved essay performance, the capacity for remote testing, and the elimination of misinterpretations, often associated with visual reading essays.
The Quantum ™ SARS-CoV-2 Ag (Fluorescence Immunoassay Antigen) test is used for the rapid and qualitative detection of the SARS-CoV-2 nucleocapsid protein antigen in a nasopharyngeal or sputum sample.
The test is for in vitro diagnostic use for professional use only. The Quantum SARS Antigen FIA test provides automated and objective results in 15 minutes, allowing sampling in patients with suspected COVID-19/2019-nCoV or people without symptoms or other epidemiological reasons to suspect they have an infection.
Comparative vs. a conventional ICA antigen test
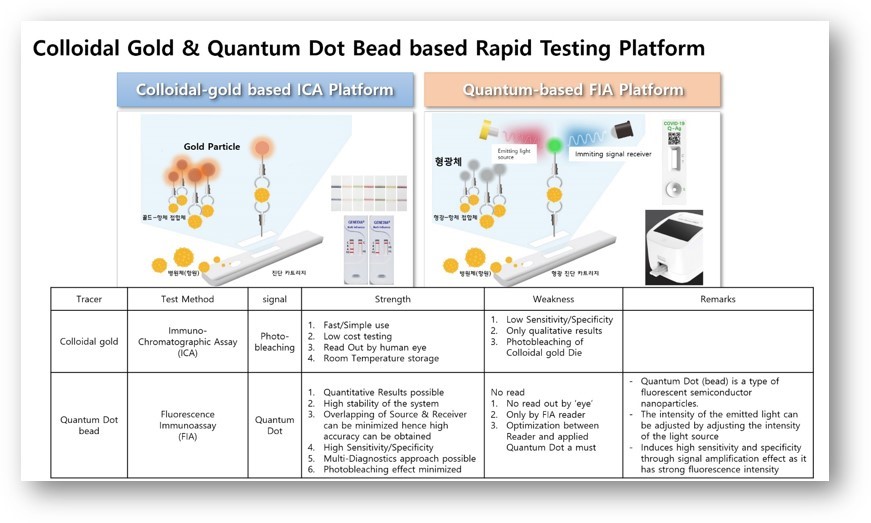
Advantages: ICA vs FIA method
When a fluorescent detection system is combined with a powerful (but inexpensive and portable analyzer such as the FR101 analyzer) the result entails better test performance, the chance to perform remote testing, as well as eliminating misinterpretations often associated with point-of-care visual reading essays.
When the strip is inserted into the analyzer, the analyzer automatically scans two tapes and detects the fluorescence intensity of the composite emission from the test area and control area.
The relationship between the two fluorescence values is used to calculate the content of the substances detected. The FR101 analyzer together with the Quantum AG test kits manage the feature that the greater the displacement or difference in the wavelength, the less interference there will be when detecting the excitation light as part of the emission light, increasing reliability and allowing the implementation of a high-sensitivity immunoassay system.
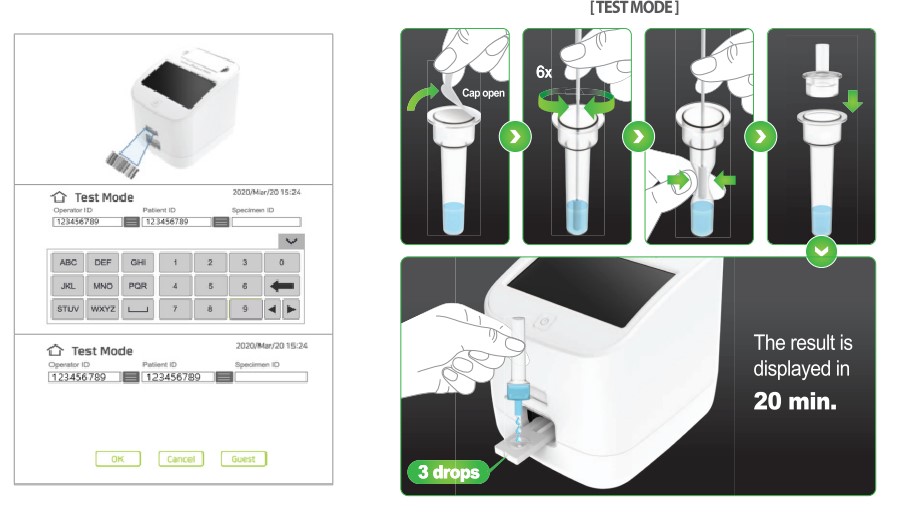
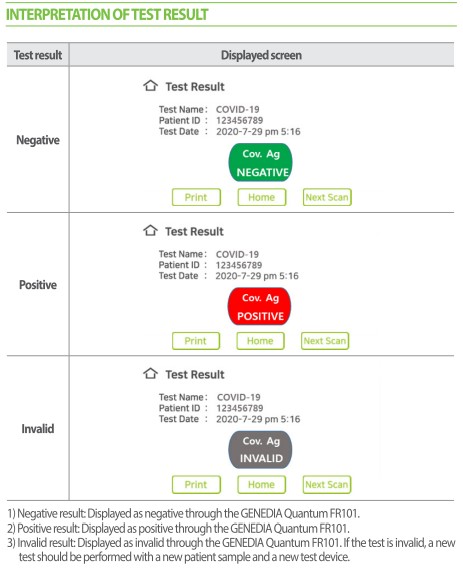
How this quantitative antigen test is performed
To perform this quantitative antigen test, follow these steps:
- The healthcare professional collects a sample from the patient using a nasopharyngeal/nasal/oral swab.
- It is then inserted into the lysis tube, stirred and drained.
- The dropper filter is then inserted in the tube, and it must be safely and carefully squeezed to filter the sample.
- 35 μ L are collected from the sample and deposited in the Covid-19 Quantum cartridge deposit.
- Finally, the sample is inserted into the machine cartridge and you must wait 10-15m to obtain reliable and accurate quantitative results.
CHARACTERISTICS
- The Quantum SARS-CoV-2 Ag FIA test is based on fluorescence immunochromatographic technology.
- Each test device has one line of anti-SARS-CoV-2 monoclonal antibody in the detection line (T line) and one line of anti-IgG polyclonal antibody in the quality control line (line C).
- When the extracted sample is added correctly to the sample, it will react with the fluorescence-labeled antibody to form a compound, the mixture then migrates across the membrane by capillary action and interacts with the anti-SARS-CoV-2 monoclonal antibody coated in line detection.
- If the sample contains SARS-CoV-2 antigen, the fluorescence compound flows through the membrane, is captured by the SARS-CoV-2 antibody, the fluorescence signal strength reflects the amount of SARS-CoV-2 captured and is detected by the Quantum ANALYZER FR101 to show a positive result.
- Otherwise, the test result will be negative.
OPERATION STEPS
- Sample collection.
- Sample preparation.
- Apply the sample.
- Read the test result.
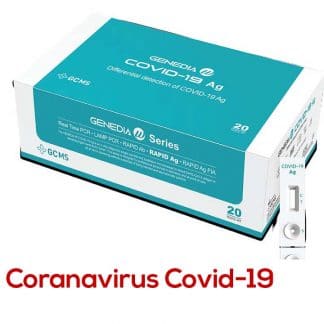
INTERPRETATION OF RESULTS
- Positive: Positive test for SARS-CoV-2 (antigen detected).
- Negative: Negative test for SARS-CoV-2 (no antigen detected).
- Invalid: Invalid test, repeat the test.